Author:scbb
Time:2014-01-13 11:26:10
Hits:
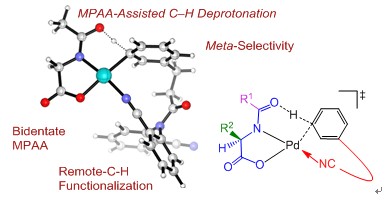
Direct activation of C–H bonds provides a sustainable and efficient methodology to synthesize diverse organic molecules from simple hydrocarbon derivatives. Despite substantial progress in this active field, improving reactivity and selectivity is a long-time goal. Recently, Yu group discovered that simple, commercially available mono-N-protected amino acid (MPAA) ligands lead to improved yield, shorter reaction times, and excellent regio- and enantioselectivities. Numerous applications using MPAA ligands to enable or improve a diverse range of C–H activation reactions with different substrate classes have appeared in the literatures. However, the role of MPAA is still not clear.
Professor Yun-Dong Wu’s group conducted a combined experimental/computational study on the amino acid ligand-assisted Pd-catalyzed C −H bond activation reveals a mechanism in which the amino acid acts as both a dianionic bidentate ligand and a proton acceptor. This new model explains the effects of amino acids on reactivity and selectivity and unveils the dual roles of amino acids: stabilizing monomeric Pd complexes and serving as the internal base for proton abstraction. The computational findings provide guidance for further improvement and development of new C–H activation reactions. This work was recently published at J. Am. Chem. Soc., (DOI: 10.1021/ja411683n).
This work is cooperated with professor Houk’s group from UCLA and professor Yu’s group from Scripps. The research efforts were carried out by graduate students Gui-Juan Cheng, Dr. Yun-Fang Yang, Ping Chen and Tian-Yu Sun at PKUSZ.